Welcome to the official website of Qingdao Puren Instrument Co., Ltd
Chromium is a kind of natural metal elements, mainly in trivalent and hexavalent two forms exist, of which hexavalent chromium easily through the cell membrane is absorbed by the body, the human body lung, liver, kidney and other organs damage, concentration Large can lead to death [1,2]. The establishment of a method for the determination of hexavalent chromium in drinking water and surface water is of great significance to protect the environment and maintain human health.
In the current method for the determination of hexavalent chromium, China's national standard method uses diphenylcarbazide derivative spectrophotometry, which has some error for the darker samples [3,4]. There are also a number of methods for the separation of hexavalent chromium and diphenylcarbazide columns after anion exchange. [5,6]. Compared with spectrophotometry, anion exchange separations can separate CrO42- from other anions and A certain enrichment effect. Accordingly, the author established a method for ion-exchange separation-diphenylcarbazide-derived UV-visible detection, which is more sensitive than the national standard method.
1 experimental part
1.1 The main instruments and reagents
Ion chromatography: PIC-10A type, Qingdao Pu Ren Instrument Co., Ltd., with UV-1100 UV - visible detector;
Analytical balance: 0.1 mg accuracy, Sartorius, Germany;
Solid phase extraction column, 0.22μm filter: Qingdao Pu Ren Instrument Co., Ltd.;
Ammonium sulfate, ammonia, potassium dichromate, dibenzoyl hydrazine: analytical grade, Shanghai Erbi Chemical Reagent Co., Ltd.;
98% concentrated sulfuric acid: analytical grade, Laiyang both chemical reagents plant;
Methanol: Chromatography Pure: Shanghai Ebi Chemical Reagent Co., Ltd.;
The experimental water is ultrapure water with a resistivity greater than 18.2 MΩ · cm.
1.2 chromatographic conditions
Column: PR-SA-8A anion exchange column (4.6 mm × 200 mm); eluent: 250 mmol / L ammonium sulfate +100 mmol / L ammonium hydroxide solution; flow rate: 1.50 mL / min; injection volume: 375 ML: Derivative: 2.0 mmol / L Diphenylcarbazide + 10% Methanol + 1.0 N Sulfuric acid, Derivative flow rate: 0.50 ml / min, reaction tube volume: 750 ml, detection wavelength: 540 nm.
1.3 Sample pretreatment
Colorless samples were filtered by 0.22 mm filter and analyzed by direct injection. Color samples were filtered and analyzed by RP column and 0.22 mm filter.
2 Results and discussion
2.1 Optimization of chromatographic conditions
Hexavalent chromium in aqueous solution generally HCrO4-, CrO42- or Cr2O72-form exists, and the solution PH has a greater relationship. In the alkaline (PH> 9) conditions, mainly in the form of CrO42-, in the conventional anion exchange column strong retention, retention time and peak type tailing. The use of high concentration of sulfate ions as eluent can significantly shorten the retention time of CrO42- and the peak type is more symmetrical. In order to keep the mobile phase alkaline, NH4 + -NH3 buffer system, so the eluent with 250mmol / L ammonium sulfate +100 mmol / L ammonium hydroxide solution.
The detection of CrO42- can be carried out by inhibition of conductivity detection, direct UV detection, and dibenzoyl hydrazine derived visible light detection. The detection sensitivity and selectivity of dibenzoyl dihydrazine derivatives are high [5,6]. The results showed that the absorbance and the concentration of hexavalent chromium were in the range of 0.01 ~ 1.0mg / L under the condition of 375ml injection volume, and the correlation coefficient was R2 = 0.9995. The detection limit of hexavalent chromium was 2.8mg / L, so the use of dibenzoyl diglyhydrazine derivative method.
2.2 Linear Equations and Quantitative Limits
The resulting sample was analyzed by adding an ion chromatograph under the determined chromatographic conditions. The results are shown in Fig.
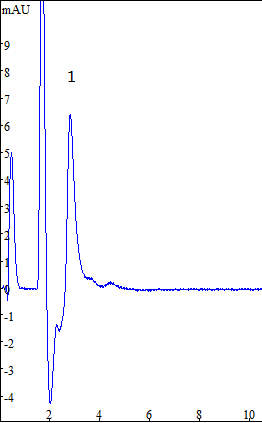
Fig.2 Ion chromatogram of hexavalent chromium in feed
(1-hexavalent chromium: 50 mg / L;)
The samples of 0.01, 0.02, 0.05, 0.2 and 1.0 mg / L hexavalent chromium were prepared according to the order of concentration from low to high. The mass concentration (mg / L) was the abscissa and the peak area was vertical Coordinate plotting linear equations. The detection limit is calculated at a signal-to-noise ratio of 3 times (S / N = 3), and the limit is calculated at a signal-to-noise ratio of 10 times (S / N = 10). The linear range, linear equation, correlation coefficient and quantitative limit of hexavalent chromium are shown in Table 1.
Table 1 Linear range, linear equation, correlation coefficient, and detection limit and quantitation limit
|
Linear range /(mg·L-1) |
Linear equation |
Correlation coefficient |
The limit of quantitation/(mg·L-1) |
The detection limit |
|
|
This article |
National standard |
||||
|
0.01-1.0 |
Y=3229000X-15540 |
0.9995 |
9.6 |
2.8 |
4.0 |
The detection limit of hexavalent chromium is 2.8mg / L, which is lower than the detection limit of 4.0 mg / L in the national standard. Therefore, the method is more sensitive than the national standard method, and the injection volume can be lower. Limit.
2.3 Recovery and reproducibility
Under the known chromatographic conditions, a certain amount of hexavalent chromium was added to the water sample for recovery test and 5 times in parallel. The test results are shown in Table 2. From Table 2, it can be seen that the recovery of hexavalent chromium is between 98 and 108%, indicating that the measurement accuracy is high. The relative standard deviation of the five determinants is between 2.63 and 4.95%, which indicates that the method has high precision and can be applied to the analysis and analysis of the actual sample.
Table 2 hexavalent chromium plus standard recovery test results
|
|
Background/(mg·L-1) |
Add value/(mg·L-1) |
Measured value/(mg·L-1) |
Recovery rate/% |
RSD/% |
|
Pond water |
ND |
50.0 |
54.0 |
108 |
2.63 |
|
Tap water |
ND |
50.0 |
49.0 |
98 |
4.95 |
3 Conclusion
The determination of drinking water and hexavalent chromium in surface water by ion chromatography column was better than that of national standard method. The analysis time was short, which was the determination of drinking water and hexavalent chromium in surface water. Chrome reliable method.
references
[1] Chen Chunding. Leather Chemicals, 2003,20 (4): 24-27
[2] He Jie, Yu Jiasheng, Huang Zhongping, et al. Analytical Chemistry, 2014,42: 1189-1194
Standard Test Method for Drinking Water of Life - Metals
[4] Yu Ruipeng, Hu Zhongyang, Ye Mingli, et al. Chromatography, 2012,30 (4): 409-413
[5] http://www.dionex.com/en-us/webdocs/4231-AN-80-IC-Chromium-Drinking-Water-AN71358-EN.pdf
[6] http://www.dionex.com/en-us/webdocs/4242-AU144_LPN1495.pdf
Determination of Chromium (Hexavalent) in Drinking water and ground water by Ion Chromatography coupled with post-column derivitization
Wang Cunjin
(Qingdao Puren Instrument Co., Ltd, Qingdao 266043, China)
Abstract A method was developed for the determination of Chromium(Hexavalent) in drinking water and ground water by ion chromatography coupled with post-column derivitization. The drinking water and ground water sample was treated by RP column and filtration to obtain clear solution. Chromium (Hexavalent) in the drinking water and ground water sample was determined by ion chromatography coupled with post-column derivitization and UV-Vis detection (530nm). The absorbance after derivitization showed good linearity with concentration in the range of 0.01-1.0 mg/L and the correlation coefficient is 0.9995. The LOD for Chromium(Hexavalent) was 2.8 mg/L, Which is superior to national standard method (4mg/L,50ml sample tested) .The recovery and repeatability were in the range of 98~108% 、2.63~4.95%, respectively. The method showed higher sensitivity and could be applied to drinking water and ground water analysis.
Keywords Chromium(Hexavalent); ion chromatography; post column derivitization; drinking water;ground wate