Welcome to the official website of Qingdao Puren Instrument Co., Ltd
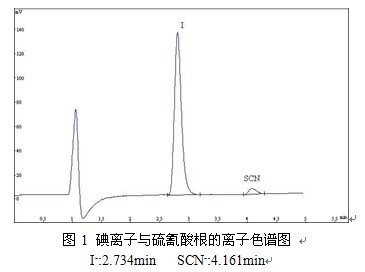
1 IntroductionMilk in the thiocyanate and iodine ions in infant food safety is an important test items. December 12, 2008 published "may be illegal in the food to add non-food substances and easy to abuse the list of food additives (first batch)" clearly defined thiocyanate is illegal addition of substances, but no corresponding GB test method. Thiocyanate has been used by unscrupulous dairy farmers because of the ability to extend the shelf life of raw milk. Excessive ingestion poses a significant threat to human health [1, 2]. Iodine is an essential element of physical and intellectual development [3,4], inadequate intake or excessive intake can cause thyroid enlargement disease. The national standard stipulates that the content of iodine per 100g milk is 30-150mg. Therefore, the determination of thiocyanate and iodide in milk is of great significance [5].At present, the use of ion chromatography on the detection of thiocyanate and iodine ions have been a number of literature, generally anion exchange separation, conductivity detection of thiocyanate, UV detection of iodine, a few electrochemical detection of two kinds of ions reported. Imported ion chromatograph manufacturers mainly involved KOH gradient leaching - inhibition of conductance or UV detection of milk thiocyanate, iodine ion [6-9], also part of the degree of leaching reported [10-12]. Li et al. [12] analyzed the iodide and thiocyanate in milk by KOH isocratic leaching-UV-conductivity tandem detection. The sensitivity of the method is higher than that of the conductivity detection method, but the sensitivity of the two methods is similar to that of the thiocyanate, and the experimental cost is high. Ye Mingli et al [9] analyzed the thiocyanate ion, iodide ion and glyphosate in drinking water by capillary elution with KOH gradient elution and inhibition conductance detection. The method was analyzed for more than 40 minutes and cost Higher. Wu Ting et al. [13] analyzed the thiocyanate in milk by using the eluent of 3.2 mmol / LNa2CO3 + 1.0 mmol / LNaHCO3 + 15% acetone (V / V), and the retention time was about 23 min.In this paper, an ion chromatography - voltammetry (single potential) method for the determination of iodide and thiocyanate was established. The separation and determination of iodide ion and thiocyanate ion were carried out in five minutes using PIC-10 ion chromatograph, NaHCO3-Na2CO3 eluent and PR-VA voltammetry produced by Qingdao Puren Instrument Co., Ltd. The detection limit of iodide was 1.9 mg / L (RSD% = 1.12%, n = 3), and the detection limit of thiocyanate was 4.7 mg / L (RSD% = 1.58, n = 3). Compared with other methods, significant savings in time, reduce costs, with a high sensitivity.2. Experimental part2.1 Instruments and reagentsInstrument: PIC-10 type ion chromatograph (Qingdao Pu Ren Instrument Co., Ltd., with Qingdao Pu Ren Instrument Co., Ltd. production PR-VA voltammetry detector) Centrifuge (Shanghai Anting Scientific Instrument Factory)Balance, precision 0.1mg (Germany Sartorius company) PG-2010 anti-control chromatography workstation, solid phase extraction column 0.22μm needle filter are Qingdao Pu Ren Instrument Co., Ltd. productionReagents: KSCN, KI, KCl, NaNO3 as excellent grade pure, CH3COOH, NaOH for the analysis of pure, ultra-pure water(Resistivity 18.2 MΩ / cm)Iodide standard stock solution: 1000mg / L, accurately weighed 0.6540g dry KI solid in 50ml glass beaker, ultra-pure water dissolved to 500ml volumetric flask, with ultra-pure water several times to wash the inner wall of the beaker, cleaning fluid the same Transferred to the volumetric flask, the final volume to 500ml.Thiocyanate standard stock solution: 1000mg / L, accurately weighed 0.8365g dry KSCN solid in 50ml glass beaker, ultra-pure water dissolved and transferred to 500ml volumetric flask, with ultra-pure water several times to wash the inner wall of the beaker, cleaning fluid The same transfer to the volumetric flask, the final volume to 500ml.2.2 chromatographic conditionsColumn: PR-SA-10A (250 mm x 4.6 mm), eluent: 12 mmol / L NaOH + 3.0 mmol / L Na2CO3, flow rate: 1.5 mL / min. Detector: PR-VA voltammeter. The voltammeter electrode was used as the Ag electrode, the reference electrode was Ag / AgCl electrode (Ag / AgCl electrode was immersed in saturated KCl solution), and the counter electrode was Pt electrode. Volt detection using single-potential method, the application of voltage -120mv. Column temperature: 25 ° C.2.3 standard solution preparationRemove the iodide standard stock solution and thiocyanate standard stock solution 1.0ml, placed in 100ml volumetric flask and ultra-pure water volume. The concentration of iodide and thiocyanate in the solution was 10mg / L.The solution 0.5, 2.0, 5.0, 10.0 and 25.0 ml were removed in 5 100 ml volumetric flasks and fixed with ultrapure water. A series of mixed standard solutions of iodide and thiocyanate were obtained.2.4 Sample pretreatmentTake 10mL of a brand of liquid milk in the centrifuge tube, add 10mL 25% CH3COOH solution, 3000r / min centrifugal 15min, put it aside after 5min supernatant to solid phase extraction column with 0.22μm needle filter after filtration analysis The3. Results and discussion3.1 The linear relationship between iodide and thiocyanate, the minimum detection limit and reproducibilityIn the determined ion chromatographic conditions, 1.0 mg / L iodide ion and thiocyanate ion standard sample into the ion chromatograph, chromatogram shown in Figure 1.
After the pretreatment of milk samples into ion chromatography, the actual sample of the ion chromatogram shown in Figure 2.
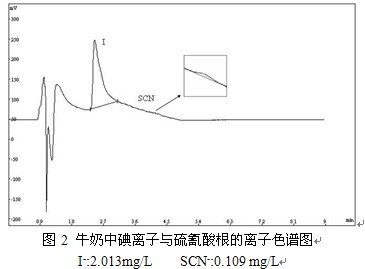
It can be seen from Figure 1, the standard sample iodide ions and thiocyanate completely separated, peak shape symmetrical, to meet the needs of chromatographic analysis. Figure 2 shows that the milk sample contains 2.013 mg / L of iodide and 0.109 mg / L of thiocyanate. According to the method of 2.4 parallel treatment of milk samples 3 times, 3 samples into the ion chromatography system, iodide ion and thiocyanate ion peak area relative standard deviation RSD% were 1.12 (I -), 1.58 (SCN-).A series of concentrations of iodide ion and thiocyanate ion standard solution were sequentially sampled to calculate the minimum detection limit at 3 times the signal to noise ratio (S / N = 3). The linear range, linear equation, correlation coefficient and minimum detection limit (n = 3) of the two ions are shown in Table 1.Table 1 Linear range, linearity, correlation coefficient and minimum detection limit of iodide and thiocyanate
|
|
Linear range(mg/mL) |
Linear equation |
Correlation coefficient / R2 |
Minimum detection limit / (mg / L) |
|
I- |
0.05-2.5 |
Y=748715.5X-5224 |
0.9994 |
1.9 |
|
SCN- |
0.05-2.5 |
Y=57251X-4490 |
0.9997 |
4.7 |
Note: Y: area of chromatographic peaks; X: ion concentration (mg / mL)
3.2 Spike recovery rate
Under the known chromatographic conditions, a certain concentration of iodine ions and thiocyanate were added to the treated milk samples for the experiment, and the samples were measured three times in parallel. The experimental results are shown in Table 2.
Table 2 iodide ion and thiocyanate plus standard recovery test results
|
|
Add value(mg/mL) |
Measured value(mg/mL) |
Recovery rate(%) |
Average recovery(%) |
|
I- |
1.0 |
2.947 |
97.8% |
98.3% |
|
1.0 |
2.898 |
96.2% |
||
|
1.0 |
3.037 |
100.8% |
||
|
SCN- |
0.1 |
0.192 |
91.9% |
93.6% |
|
0.1 |
0.200 |
95.7% |
||
|
0.1 |
0.195 |
93.3% |
t can be seen from Table 2 that the method has good recovery rate for iodide and thiocyanate, and is suitable for the analysis and analysis of the actual sample.
4 Conclusion
In this paper, ion chromatography-voltammetry was used to analyze the separation of two ions in milk samples (I-2.013mg / L, SCN-0.109mg / L) in iodine ion and thiocyanate in five minutes ). Compared with other existing methods, significant savings in time and reduce costs. (RSD = 1.12%, n = 3). The detection limit of thiocyanate was 4.7 mg / L (RSD = 1.58%, n = 3), and the detection limit of thiocyanate was higher The sensitivity and recovery rate provide a feasible method for the daily detection of iodide ions and thiocyanate ions in milk.
5. References
1. Zhang Xiaole, Yan, Zhang Yijiang, et al., Environmental and Health Impurities, 2007. 24 (11): p. 910.
2. Zhang Guizhu, Zhang., Guo Wei, et al., Analytical Science, 1994. 10 (3): p.
3. Diane ion chromatography application technology album (Volume 2), food and beverage and drug analysis.
4. Jiang Hua, Zhou., Wang Yubao, Analytical Journal, 2004. 23 (5): p.
5. Determination of iodine in infant food and dairy products, GB 5413.23-2010.
Ministry of Health, Food & Beverage Office [2009] No.29.
Determination of Thiocyanate in Liquid Milk by Ion Chromatography. Technology * Food Engineering, 2010: p. 156-158.
8. Determination of Thiocyanate in Milk by Ion Chromatography. The Feed Industry, 2011. 32 (19): p. 43-44.
9. Ye Mingli, Hu., Pan Guangwen, Determination of Iodide, Thiocyanate and Glyphosate in Drinking Water by Capillary Ion Chromatography. Chinese Journal of Analytical Chemistry, 2011. 11 (39): p. 1762-1765.
Determination of Thiocyanate in Milk Powder and Liquid Milk by Ion Chromatography. Chinese Journal of Analysis Laboratory, 2009. 28 supply: p. 150-153.
Determination of Iodide and Thiocyanate in Milk Powder by Ion Chromatography with Mixed Mode Chromatography LI Jing, WANG., LIANG Li-na, Chinese Journal of Chromatograph, 2010 28 (4): p. 422-425.
Analysis of Iodide and Thiocyanate in Milk Powder by Ion Chromatography LI Jing, WANG., LIANG Li-na, Chinese Journal of Analytical Laboratory, 2010 29 (5): p.
13. Wu Ting, Hao., Determination of Thiocyanate in Milk by Ion Chromatography. Chinese Journal of Analysis Laboratory, 2009. 28 supply: p. 206-208.
THE DETERMINATION OF IODIDE AND THIOCYANATE IN MILK BY ION CHROMATOGRAPHY WITHVOLTAMMETRY DETECTION
Hou Qianhui, Du Xiaolei, Wang Cunjin, Zhao Aiguo
Qingdao Puren Instrument Company Limited, Qingdao 266043, China
Abstract: An ion chromatography - voltammetry (single potential) method by using China instrumentwas developed for the rapid determination of iodide (I-) and thiocyanate (SCN-) with detection limits: 1.9 mg/L , 4.7 mg/L, reproducibility (RSD %, n = 3): 1.12% , 1.58%, accuracy (recovery): 98.3%, 93.6% respectively. The concentration (I-): 2.013mg/L, (SCN-): 0.109mg/Lin milk were detected in 5minutes. The method showed higher sensitivity, better accuracy and shorter analytical period, and would be applied to milk routine analysis.
Key words: ion chromatography, voltammetry, single potential, iodide, thiocyanate