Welcome to the official website of Qingdao Puren Instrument Co., Ltd
Drinking water with chlorine disinfection, because of its low cost, good disinfection effect is widely used. However, since 1970, studies have shown that the use of chlorine disinfection in the process may produce carcinogenic by-products such as trihalomethanes, so countries are looking for a more safe and reliable alternative to chlorine disinfection methods. Currently developed countries are more common use of chlorine dioxide and ozone as drinking water and water tank disinfectant. Studies have shown that the above disinfectants in the course of the use of a small amount of adverse health products such as, chlorite, chlorate and bromate. The use of chlorine dioxide solution for drinking water can be accompanied by the formation of chlorite (ClO2-) and chlorate (ClO3-). With hypochlorite (such as bleach) solution disinfection, will lead to the end of the treatment of drinking water. And the use of ozone on the process of drinking water disinfection of water will naturally exist in the bromide oxidation of harmful to the human body of bromate. As a by-product of ClO2-disinfectants, many reports have been reported on ClO2- and ClO3-. The use of chlorine dioxide on drinking water disinfection, chlorine dioxide dissolved in water, the formation of chloric acid and chloric acid.
2ClO2 + H2O → HClO2 + HClO3 studies have shown that ClO3 is stable in drinking water and ClO2 can be lost in 24 hours. ClO2- can react with hypochlorite (ClO-) to form an aqueous solution of the filter.
The ions that are often required to be measured in drinking water include the common byproducts of the anions, cations and disinfectants such as some halogen oxyacids: chlorite (ClO2-), hypochlorite (ClO-), chlorate (ClO3-), Bromate (BrO3-) and the like. In the water treatment process, the amount of chlorine dioxide used is very small, so the water reaction byproduct chlorite and chlorate concentration is usually relatively low. In general, the use of imported anion column, large volume injection sample direct determination of drinking water in low concentrations of halogen acid and bromate, in order to make the baseline noise in a smaller state, after the start of the instrument to stabilize the time to be slightly longer, So that the background conductance reaches a steady state.
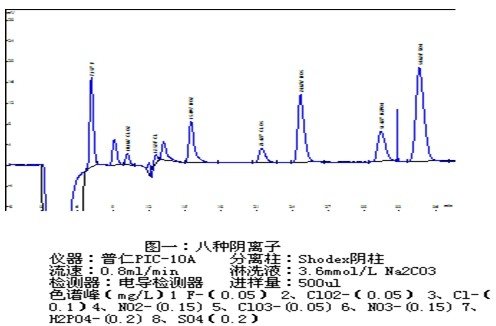
Although bromate has been used in beverage and bread manufacturing for many years, recent studies have shown that bromate is a potential carcinogen. Ozone can cause the attention of bromide in the drinking water to oxidize bromate that affects human health. The World Health Organization recommends that the content of bromate in drinking water should be controlled below 25 μg / L.
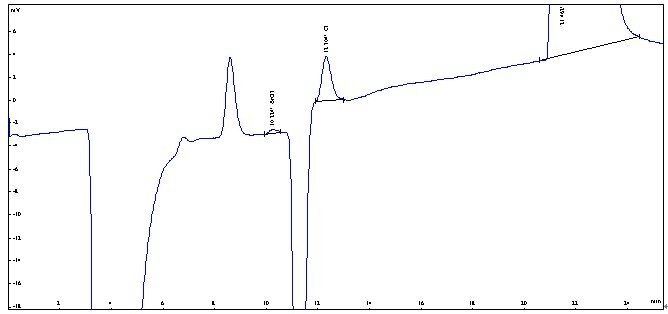
In the case of sample analysis, high levels of chlorine on the low content of bromate ions in the presence of interference, need to first through the Ag column to remove the chloride ions, and then injection. The next picture is a time ago to Hangzhou, a customer to do the actual sample.