Welcome to the official website of Qingdao Puren Instrument Co., Ltd
1 principle
Hydrogen chloride and hydrochloric acid in the air were collected by a porous glass plate with an alkaline solution, separated by column separation, conductivity detector, retention time, peak height or peak area.
2 instrument: porous glass plate absorption tube; air sampler, flow 0 ~ 1L / min; microporous membrane, aperture 0.2μm; filter device; with a scale test tube, 5ml; Qingdao Pu Ren Instrument Co., Ltd. PIC- Type ion chromatograph
2.1 Instrument operating conditions
Column: Anion column and its anion guard column produced by Qingdao Pu Ren Instrument Co., Ltd.
Mobile phase: eluent (can also be used to absorb liquid), the experimental solution for the NaOH, it is recommended to use after the eluent as the absorption solution. Causing the water to be smoothed at the peak.
Flow rate: 1.3ml / min.
3 reagent
The experimental water is deionized water.
3.1 Absorbent liquid (mobile phase): Weigh 0.204g sodium carbonate and 0.151g sodium bicarbonate diluted to 1L with water, shake evenly.
3.2 standard solution: Weigh 0.2044g potassium chloride (dried at 110 ℃ 2h), dissolved in water, quantitative transfer into 1000ml
Capacity bottle, diluted to the scale. The solution was 100 μg / ml of standard stock solution. Before use, diluted with the absorption solution into 10.0g / ml hydrogen chloride standard solution. Or with the national standard solution approved.
4 sample collection, transportation and preservation
Site sampling is performed according to GBZ 159.
At the sampling point, a 15 mm air sample was collected at a flow rate of 1 L / min with a perforated glass plate with a 5.0 ml absorbent solution. Immediately after sampling, the inlet and outlet of the absorption tube are closed; the cleaning container is transported and stored, and the sample can be stored for 7 days at room temperature.
5 Analysis steps
5.1 Control test: A porous glass plate with 5.0 ml of absorption liquid was taken to the sampling point. The air samples were collected without the air sampler. The remaining samples were the same as the blank control of the sample.
5.2 Sample treatment: with the absorption tube in the absorption tube to absorb the absorption tube into the inner wall of the pipe 3 times, with a microporous filter filter into the plug scale tube for the determination. If the concentration of the sample in the sample solution exceeds the measurement range, it can be diluted with the absorption solution and calculated by multiplying the dilution factor.
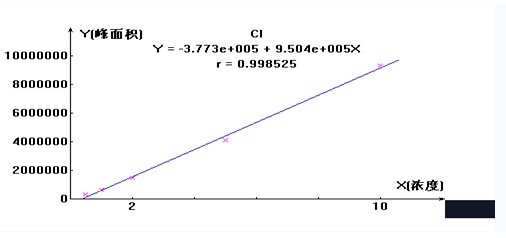
5.3 standard curve drawing: take four plug scale test tube, respectively, by adding 0.0,0.25,0.75,1.25ml
Hydrogen chloride standard solution, each add liquid to 5.0ml, dubbed 0.0,0.50,1.50,2.50g / ml
Hydrogen Chloride Standard Series. According to the operating conditions of the instrument, the ion chromatograph was adjusted to the best measurement conditions, 50ml injection, respectively, the standard series, the concentration of each repeated determination of 3 times, the peak height or peak area of the corresponding concentration of hydrogen chloride (g / ml) Draw a standard curve.
5.4 Sample determination: Determination of the standard series of operating conditions for the determination of sample solution and sample blank control solution. The peak or peak area of the sample is subtracted from the peak or peak area of the sample blank control and the concentration of hydrogen chloride (g / ml) from the standard curve.
5.5 Spectrum
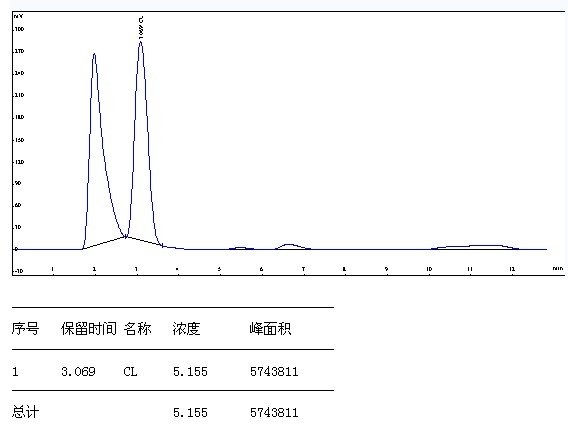
6 calculation
6.1 The sample volume is converted into the standard sampling volume according to equation (1).
6.2 Calculate the concentration of hydrogen chloride in the air according to formula (3)
5 c
C = ------ (3)
Vo
Where:
C - concentration of hydrogen chloride in air, mg / m3;
5 - the volume of the absorbed liquid, ml;
C - measured concentration of hydrogen chloride in the sample solution, g / ml;
Vo - standard sampling volume, L.
6.3 Calculated results: 0.00172 g / ml.
7 Description: GB detection limit of 0.08g / ml; the lowest detection concentration of 0.027mg / m3 (to collect 15L air samples). The determination range is 0.08 ~ 2.5g / ml; the relative standard deviation is 3.0% ~ 3.3%. The content of hydrogen chloride in the area was lower than the detection limit.